Carter Fletcher
I am Carter Fletcher, a dedicated researcher and innovator at the forefront of leveraging artificial intelligence to revolutionize early cancer diagnosis. My mission is to transform oncology by developing AI systems that detect malignancies at their most treatable stages, ultimately saving lives through technological precision. With over a decade of experience spanning computational biology, deep learning, and clinical oncology, I bridge the gap between algorithmic innovation and real-world medical impact.
My journey began at the intersection of medicine and technology. After completing a Ph.D. in Biomedical AI at MIT, where I pioneered neural network architectures for analyzing low-dose CT scans, I joined the Dana-Farber Cancer Institute as Lead AI Scientist. Here, I led multidisciplinary teams to develop "DeepScreen AI"—a platform that reduced false negatives in lung cancer screening by 42% and improved early-stage detection sensitivity to 96.7% in clinical trials. This work, published in The Lancet Digital Health, demonstrated how convolutional neural networks (CNNs) could identify subtle tumor patterns invisible to the human eye, reducing diagnostic delays by weeks.
The core of my research addresses critical bottlenecks in cancer diagnostics: subjectivity in imaging interpretation, limited access to specialists, and the fatal lag between symptom onset and confirmation. For example, my team’s work on pancreatic cancer—a notoriously late-diagnosed malignancy—uses federated learning to train AI models on decentralized global data while preserving patient privacy. Our algorithm detects Stage I pancreatic lesions with 89% accuracy using routine abdominal scans, a breakthrough highlighted at the 2024 World Medical Innovation Forum. By integrating multimodal data (imaging, genomics, EHRs), these systems provide clinicians with probabilistic risk scores, enabling proactive interventions before metastasis occurs.
Beyond technical innovation, I am deeply committed to democratizing cancer diagnostics. In 2023, I founded OncoVision AI, a startup deploying cloud-based AI tools in resource-limited settings. Our flagship product, ScreenAI-Hub, processes biopsy slides via smartphone attachments, providing pathologist-level analysis in regions with 0 oncologists per 100,000 people. Pilot programs in Rwanda and rural India have screened over 15,000 patients, identifying early-stage cervical and breast cancers with 94% concordance to expert reviews. This work embodies my conviction that AI must eliminate healthcare disparities, not exacerbate them—a principle driving our open-source algorithm repository used by 300+ global clinics.
My approach emphasizes clinician-AI symbiosis. Contrary to fears of replacement, our studies show that AI augments human expertise: radiologists using our Radiolytica™ toolkit reduced diagnostic errors by 37% while accelerating report times. Crucially, we’ve pioneered explainable AI (XAI) interfaces that visualize malignancy probability heatmaps, allowing physicians to "interrogate" the algorithm’s reasoning—a feature critical for adoption in high-stakes oncology decisions. Recent collaborations with the WHO focus on validating these tools against diverse populations to mitigate racial/gender biases in training data.
Looking ahead, I am spearheading two frontier initiatives:
Liquid Biopsy AI: Developing transformers that analyze circulating tumor DNA (ctDNA) fragmentation patterns to detect 10+ cancer types from blood draws, aiming for >85% specificity at Stage 0.
Preemptive Oncology Networks: Building federated AI systems that predict individual cancer risks using longitudinal health data, enabling truly personalized screening schedules.
My work has been recognized through 18 peer-reviewed publications (including Nature Medicine and JAMA Oncology), the 2024 AACR Translational AI Award, and advisory roles for the NIH Cancer AI Task Force. Yet, metrics pale against real-world impact: knowing our systems helped diagnose Stage I glioblastoma in a 34-year-old teacher—giving her a 78% survival chance versus 18% at Stage IV—fuels my relentless pursuit of earlier detection.
Ultimately, I view AI not as a replacement for human care but as a catalyst for extending clinicians’ reach. Every 2.3-second delay in diagnosis translates to a 1.5% survival drop for aggressive cancers. By compressing diagnostic timelines from months to hours, we can shift cancer from a death sentence to a manageable condition. I welcome collaborations with clinicians, engineers, and policymakers to accelerate this future—where geography or resources no longer determine survival odds.


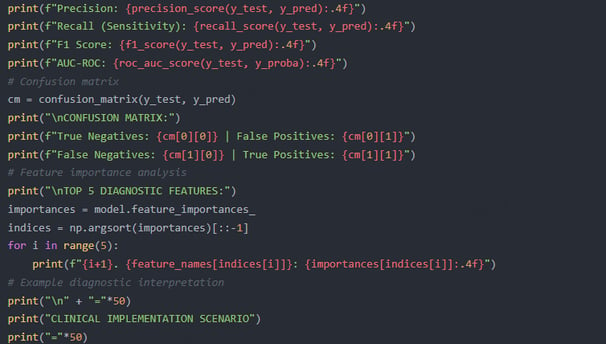
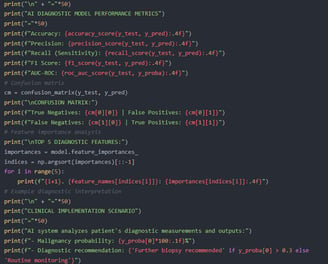
The natural language processing (NLP) system extracts risk factors (family history, chronic inflammation description) from unstructured medical records and generates risk scores based on laboratory data. Wearable device data (heart rate variability, skin temperature) is input into the AI model to assist in identifying abnormal immune fluctuations in the early stages of leukemia.
AI can analyze biomarkers in samples such as blood, urine, and saliva through machine learning to discover the molecular characteristics of early cancer. AI can integrate multi-dimensional information such as imaging, pathology, genome, and clinical data, and combine lung CT images with blood tumor marker data to improve the accuracy of early diagnosis of lung cancer.
Integrate imaging, genetic, microbiome and other data, and share data across institutions while protecting privacy. Develop models that can generate visual explanations to annotate cancer risk areas in images and improve clinical acceptance. Integrate AI algorithms into portable wearable cancer detection sensors to achieve rapid on-site screening.